Innovative. Flexible. Efficient.

Flexible solutions for pharmaceutical processing
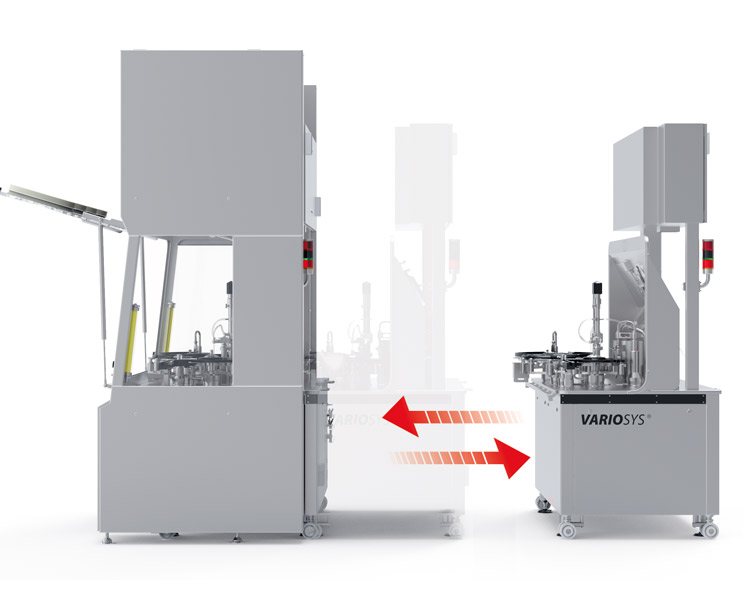
The innovative and highly flexible VarioSys® production system for biotech and pharmaceuticals is essentially a combination of two elements: an isolator made by SKAN and machine modules made by Bausch+Ströbel. The system has been designed to provide utmost flexibility in the production of medications by exchanging modules. The machine modules are simply slotted into place on the "lock-and-key" principle and plugged in. Pharmaceutical production can be extended by adding a suitable freeze-dryer made by GEA.
VarioSys® is suitable for a wide range of applications that the manufacturing of clinical samples as well as the production of batches is possible in one installation. All FDA requirements for pharmaceutical production are met.

Fields of application
Maximum flexibility for small batch aseptic manufacturing and laboratory applications in the isolator
- Commercial manufacturing
- Clinical filling
- Filling personalized medicines
- Flexible contract manufacturing
- Small batch manufacturing
- Product development
- Start-up / Scale-up
- Process development
Benefits

Plug and Play

Adaptable to various packaging materials

Extendable modular design

Modular design of the processing line

Lock and key

Standardization of subassemblies

GMP and FDA conforming machine design

Processing highly potent medications
Space saving

Time efficient
Advanced Aseptic Pharmaceutical Production in a Scalable Isolator
Abstract
This article describes a new filling and closing module for syringes and cartridges for biotech and pharmaceuticals, working aseptically in a modular barrier isolator with an automated H2O2 decontamination system. The isolator has a modular structure. Both the isolator modules and the machine modules can be combined depending on the production requirements. The isolator modules can be used in aseptic as well as in aseptic toxic production.
Advanced Aseptic Pharmaceutical Production in a Scalable Isolator

Longstanding partnership between Bausch+Ströbel and SKAN
 Just like the perfect blend of milk and coffee in a cappuccino, our longstanding partnership with SKAN is a harmonious collaboration that has matured beautifully over the years. This video is a testament to the enduring qualities of teamwork, dedication, and the art of working together that have been at the heart of our relationship.
Just like the perfect blend of milk and coffee in a cappuccino, our longstanding partnership with SKAN is a harmonious collaboration that has matured beautifully over the years. This video is a testament to the enduring qualities of teamwork, dedication, and the art of working together that have been at the heart of our relationship."Longstanding partnership between Bausch+Ströbel and SKAN" auf YouTube anschauen

VarioSys World
 Episode 1: How it works
Episode 1: How it works
In this episode, Ryan and Alex introduce GRAM’s VarioSys technology and explain the benefits of modular design and multi-component flexibility.Watch "VarioSys World Episode 1" on YouTube

 Episode 2: Containment
Episode 2: Containment
A special guest joins Alex and Ryan in episode two to discuss how GRAM’s flexible filling equipment maintains aseptic processing and a quality environment while staying flexible.Watch "VarioSys World Episode 2" on YouTube

 Episode 3: Maximum Yield
Episode 3: Maximum Yield
Ryan and Alex welcome a special guest to talk about advanced aseptic filling technology, line loss minimization, in-process checks, and more included in GRAM’s flexible filling equipment.Watch "VarioSys World Episode 3" on YouTube

 Episode 4: Customization
Episode 4: Customization
GRAM experts discuss how innovative modular technology allows for unique customization to meet a multitude of customer needs in this episode.Watch "VarioSys World Episode 4" on YouTube

Evonik White Paper – VarioSys® Aseptic Filling Line
- Production of complex parenterals
- Flexibility during production
- Different in-line dosing processes for liquid and powdered products
- Simultaneous cost and time reduction
Evonik White Paper – VarioSys® Aseptic Filling Line

